Home > Structure > RNA Biochemistry Research Unit > Zebrafish Developmental Neurobiology
Zebrafish Developmental Neurobiology

Savani Anbalagan, PhD
Principal Investigator
Research
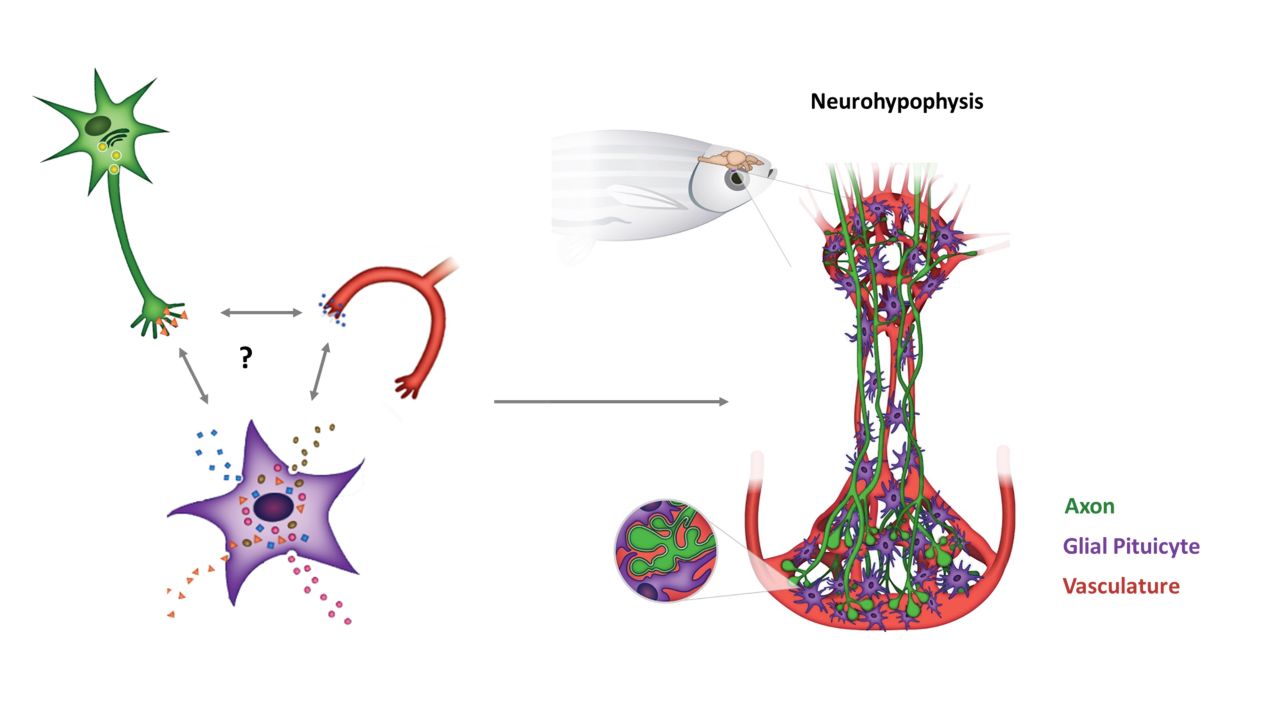
Oxytocin (OXT) is a hypothalamus-derived neuropeptide majorly secreted via the neurohypophysis (posterior pituitary). During social, feeding and reproductive behaviors, OXT is released via synapses both centrally and into the peripheral blood circulation. Reduced hypothalamic OXT content in autism-like mice models and claims of genetic polymorphisms in oxytocin receptor gene of children with social deficits led to the controversial OXT-deficit hypothesis of autism spectrum disorders (ASD). Thus, investigating the molecular mechanisms that regulate OXT content and release can aid in understanding factors underlying ASD.
At a molecular level, neurohypophyseal OXT synapses-associated glial pituicytes can secrete factors that affect OXT content or release. However, the identity and the role of pituicyte-derived secreted factors that can regulate synaptic OXT are largely unknown. Finally, situated outside the blood-brain barrier (BBB), glial pituicytes are directly exposed to the blood-borne molecules and are highly responsive to physiological challenges to promote synaptic plasticity. But the identity and the role of the pituicyte-derived cues that regulate the plasticity of OXT axonal terminals and OXT neuropeptide content upon challenges are largely unknown.
Our group combines molecular and advanced light microscopy techniques to study the role of glial pituicytes using zebrafish as vertebrate model organism.
Selected publications
- Sugar-sensing swodkoreceptors and swodkocrine signaling. Anbalagan S. Animal Model Exp Med. 2025. doi: 10.1002/ame2.70007
- Targeted blocking of gene splicing can dysregulate intron-embedded microRNAs. Ali MH, Kwiatkowski W, Kopec P, Nedunchezhian N, Pecherz S, Kowalewska N, Gnutti B, Finazzi D, Anbalagan S. bioRxiv. 2024. doi: 10.1101/2024.12.04.626772
- Temperature-sensing riboceptors. Anbalagan S. RNA Biol. 2024. doi: 10.1080/15476286.2024.2379118.
- Gas-sensing riboceptors. Anbalagan S. RNA Biol. 2024. doi: 10.1080/15476286.2024.2379607.
- Wodne akwareceptory na bazie hemu. Anbalagan S. Postępy Biochemii. 2024. doi: https://doi.org/10.18388/pb.2021_551
- Oxygen is an essential gasotransmitter directly sensed via protein gasoreceptors. Anbalagan S. Animal Model Exp Med. 2024. doi: 10.1002/ame2.12400
- Heme-based oxygen gasoreceptors. Anbalagan S. Am J Physiol Endocrinol Metab. 2024. doi: 10.1152/ajpendo.00004.2024
- “Blindmen and an elephant”: The need for animals in research, drug safety studies, and understanding civilizational diseases. Anbalagan S. Animal Model Exp Med. 2023. doi: 10.1002/ame2.12364
- A ligand-receptor interactome atlas of the zebrafish. Chodkowski M, Zielezinski A, Anbalagan S. iScience 2023. doi: 10.1016/j.isci.2023.107309
- Stress resilience is established during development and is regulated by complement factors. Swaminathan A, Gliksberg M, Anbalagan S, Wigoda N, Levkowitz G. Cell Rep. 2023. doi: 10.1016/j.celrep.2022.111973.
- Endocrine cross-talk between the gut microbiome and glial cells in development and disease. Anbalagan S. J Neuroendocrinol. 2021. doi: 10.1111/jne.12924.
- Robo2 regulates synaptic oxytocin content by affecting actin dynamics. Anbalagan S*, Blechman J*, Gliksberg M, Gordon L, Rotkopf R, Dadosh T, Shimoni E, Levkowitz G. Elife. 2019. doi: 10.7554/eLife.45650.
- Pituicyte Cues Regulate the Development of Permeable Neuro-Vascular Interfaces. Anbalagan S*, Gordon L*, Blechman J, Matsuoka RL, Rajamannar P, Wircer E, Biran J, Reuveny A, Leshkowitz D, Stainier DYR, Levkowitz G. Dev Cell. 2018. doi: 10.1016/j.devcel.2018.10.017.
- Genome Editing Reveals Idiosyncrasy of CNGA2 Ion Channel-Directed Antibody Immunoreactivity Toward Oxytocin. Blechman J, Anbalagan S, Matthews GG, Levkowitz G. Front Cell Dev Biol. 2018. doi: 10.3389/fcell.2018.00117.
More publications in PubMed.
Research grants
-
Rola intronowego mikroRNA indukowanego oligonukleotydem antysensownym w morfogenezie aksonów u danio pręgowanego
National Science Centre (SONATA 17) - Rola interakcji akson-glej, w której pośredniczy Sfrp5 w morfogenezie aksonów danio pręgowanego podwzgórzowo-przysadkowa
National Science Centre (SONATA BIS 10)